Switch?
About Alvesco.
When choosing an ICS to prescribe for your mild-to-moderate asthma patients, consider Alvesco. It’s the maintenance inhaler designed to reach deep into the lungs and treat both large and small airways.1,2,3,8 When used as prescribed, Alvesco may help patients breathe easier by reducing airway inflammation.
Treats
deep: Alvesco Inhalation Aerosol is an
ICS with small particle size (~1-2 µm)2,*
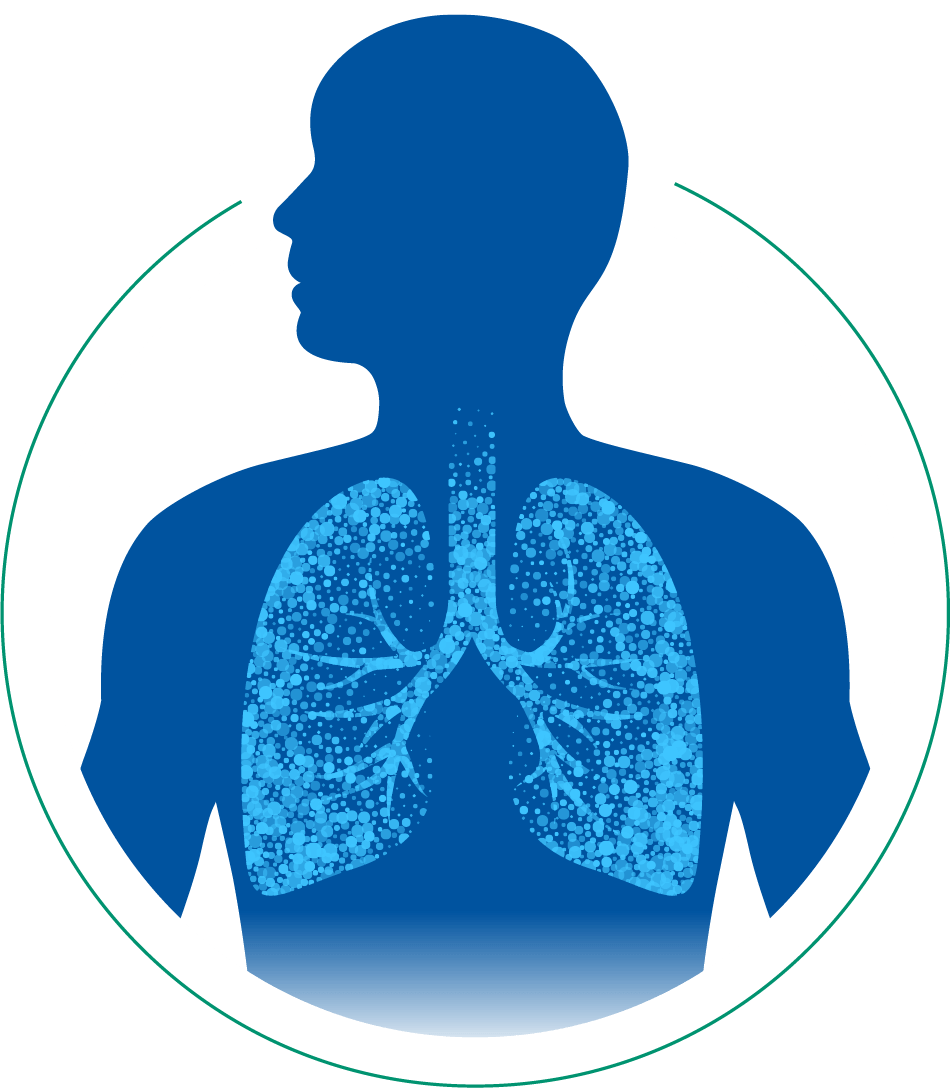
Small particle size enables Alvesco to reach areas of chronic inflammation in both the large and small airways in the lungs. Treating deep may help to maintain control of asthma symptoms.2,3
- Aerosol particle size is a key factor in both the extent of lung deposition and the regional distribution within the lung.4
- ICS particle sizes <2 µm in diameter are more likely to reach the small airways of the lungs. Here they can have a targeted effect without as many local adverse effects in the throat and oral cavities as inhalers with larger particle sizes.3,*
*Particle size does not necessarily correlate with clinical efficacy.
Favorable safety profile.
Alvesco Inhalation Aerosol was studied in 4 clinical trials with a total of 624 patients ages 12 years and older (359 females and 265 males) with asthma of varying severity.1
- Patients were treated with Alvesco 80 mcg, 160 mcg, or 320 mcg twice daily for 12 to 16 weeks.
- Patients had previously used either controller therapy (usually ICS) or reliever therapy (bronchodilator therapy alone).

In these trials, the most common adverse reactions (≥3% and more frequent than with placebo) were1:
- Headache
- Nasopharyngitis
- Sinusitis
- Pharyngolaryngeal pain
- Upper respiratory infection
- Arthralgia
- Nasal congestion
- Pain in extremity
- Back pain
Alvesco was studied in another 12-week trial in asthma patients 12 years of age and older who had previously required oral corticosteroids (average daily dose of oral prednisone of 12 mg/day).1
- Alvesco 320 mcg twice daily (n = 47) and 640 mcg twice daily (n = 49) were compared with placebo (n = 45) for the frequency of reported adverse reactions.
The following adverse reactions occurred at an incidence of ≥3% in the Alvesco-treated patients and were more frequent than with placebo1:
- Sinusitis
- Hoarseness
- Oral candidiasis
- Influenza
- Pneumonia
- Nasopharyngitis
- Arthralgia
- Back pain
- Musculoskeletal chest pain
- Headache
- Urticaria
- Dizziness
- Gastroenteritis
- Face edema
- Fatigue
- Conjunctivitis
Use in specific populations1:
Pregnancy - There are no adequate and well-controlled studies in pregnant women. Alvesco should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing mothers - It is not known if ciclesonide is secreted in human milk. However, other corticosteroids are excreted in human milk. Caution should be used when Alvesco is administered to nursing women.
Pediatric use - The safety and effectiveness of Alvesco in children under 12 years of age have not been established.
Geriatric use - Clinical studies of Alvesco did not include sufficient numbers of patients aged 65 years and older to determine whether they respond differently than younger patients. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range reflecting the greater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease, or other drug therapy.
Alvesco maintains a favorable safety profile.11
Switch patients.
A favorable safety profile affordable access programs.
Ciclesonide maintains a favorable safety profile with baseline control when switching your patients previously treated with fluticasone or beclomethasone inhaled corticosteroids.9,11