
Order Samples Now
Available in two strengths for your patients: 80 mcg and 160 mcg inhalation aerosol
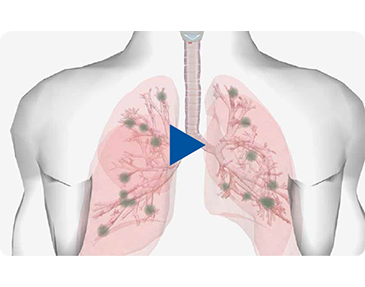
Alvesco in 3D Action
Asthmatic inflammation can occur throughout the tracheobronchial tree. See how small ICS particles (<2 µm) are more likely to reach small airways where they can exert their anti-inflammatory effect.2
Contact Us
If you have questions about ordering Alvesco samples for your patients, product availability or the Alvesco patient assistance programs, please email us at: copay@covispharma.com
Get In Touch